The great strength of 30×30, the national goal to protect 30 percent of the ocean by 2030, is that it sets out a framework wherein there is agreement that our shared use of public lands and waters fall along a spectrum, ranging from sacred places to wise use.
Our field is competitive, some job postings are confusing, and some career advice is contradictory or wrong. Here’s an exercise I have my students perform that I hope can help you. Graphic via Woods Hole Oceanographic Institution Hardly a day goes by that I don’t see a heartbreaking post from a prospective marine biologist in … Read More “Here’s what I teach my students about finding jobs in marine biology and conservation” »
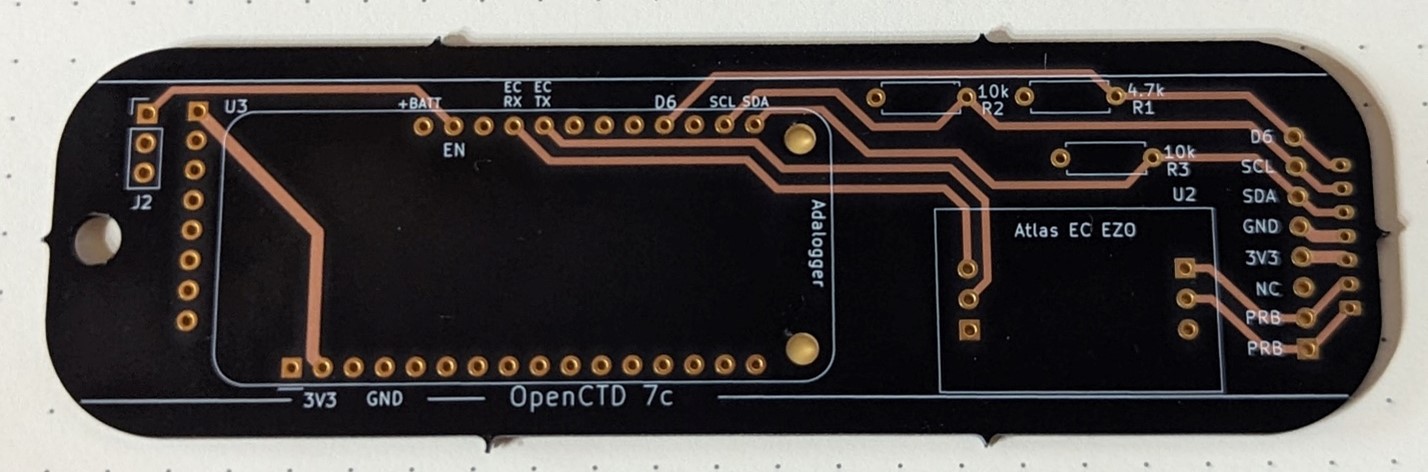
Last month, I wrote a heartwarming little story about how doing a fun weekend hardware project with my daughter led to fixing one of the most annoying non-critical problems with the OpenCTD. After a month of testing, we have fully implemented the new power management system into the next iteration of the OpenCTD. Currently, there … Read More “Charging the OpenCTD is annoying, so we fixed it.” »
After a trio of very widely read articles triggered a traffic surge in February, including David’s critical expert analysis of cross-order hybridization, our visitor count normalized a bit on the old ocean science and conservation blog. A little more than 19,500 people visited Southern Fried Science in March, a roughly 50% increase from January. You … Read More “Space Crabs, Big Boats, and Fake Sharks: What you read on Southern Fried Science in March” »
A new study shows how unique cells full of crystals give this ray one of the brightest blue colors in nature Photo of Dr. Shahrouz Amini, MPI Potsdam, photographing a ray’s blue spots Coral reef fishes come in all the colors of the rainbow (and perhaps even in some colors that we can’t see). But … Read More “How the Bluespotted Ribbontail Stingray got its Spots” »
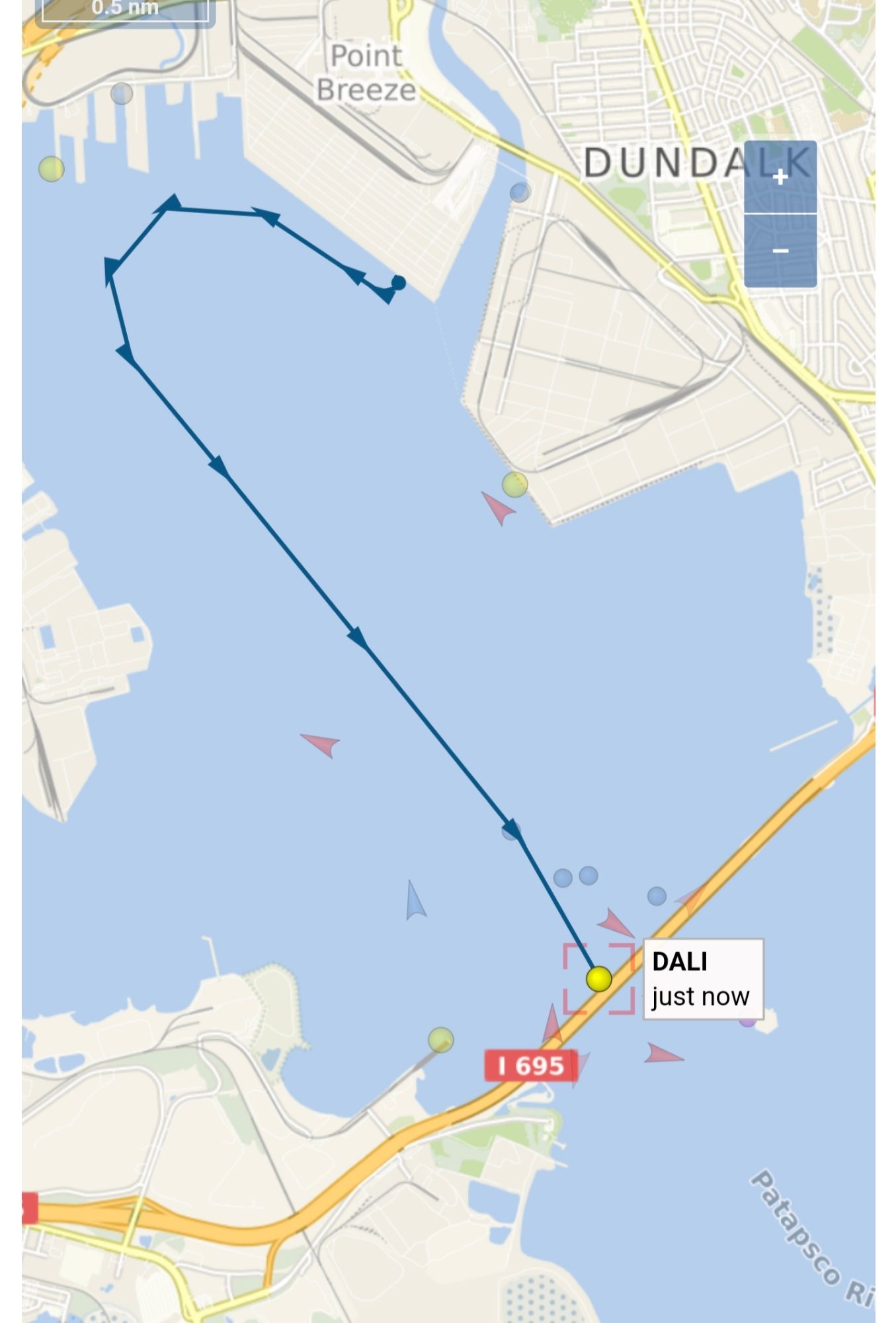
Early this morning, the cargo ship MV Dali collided with the Francis Scott Key Bridge, causing the bridge to collapse, sending several vehicles and people into the water. Search and rescue is currently underway. Because Twitter is now a clearinghouse for the worst and most disingenuous hacks on the web, there’s of course a rumor … Read More “No, the ship didn’t steer towards the pylon: A brief fact check on the MV Dali collision with Baltimore’s Key Bridge” »
The International Seabed Authority is meeting this month in Jamaica, but it is not the entire International Seabed Authority. Only the Legal and Technical Commission and the Council meet this months. The Legal and Technical Commission is a body of experts that reviews documents and proposals, usually in private as many contain privileged information from … Read More “What I’m watching for at this month’s ISA meeting: The Vibes” »
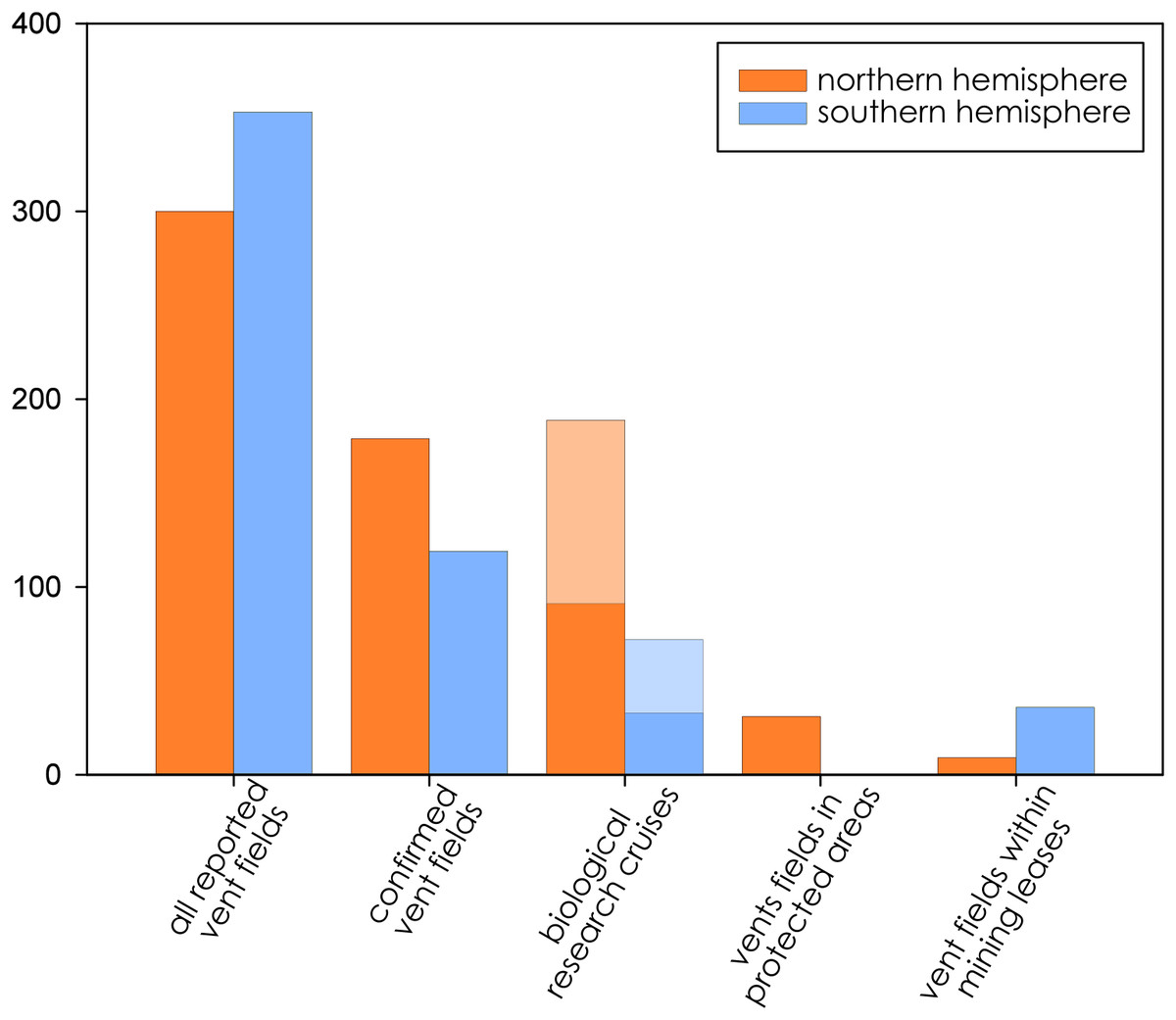
Cultural Heritage is a bit of a tough concept when working in areas beyond national jurisdiction. By definition, the places being considered for deep-sea mining by the International Seabed Authority exist at least 200 nautical miles from land and human habitation. Even most submerged archeological sites lie on continental shelves within nations’ exclusive economic zones. … Read More “What I’m watching for at this month’s ISA meeting: How to Value Cultural Heritage on the High Seas?” »
The Common Heritage of Mankind. The core principle that underlies all of the negotiations surrounding deep-sea mining beyond national borders is that these resources don’t belong to any one person, organization, or nation, but to humankind as a whole, to be exploited (or not) for the benefit of the world as a whole. With the … Read More “What I’m watching for at this month’s ISA meeting: untangling the financial regime” »
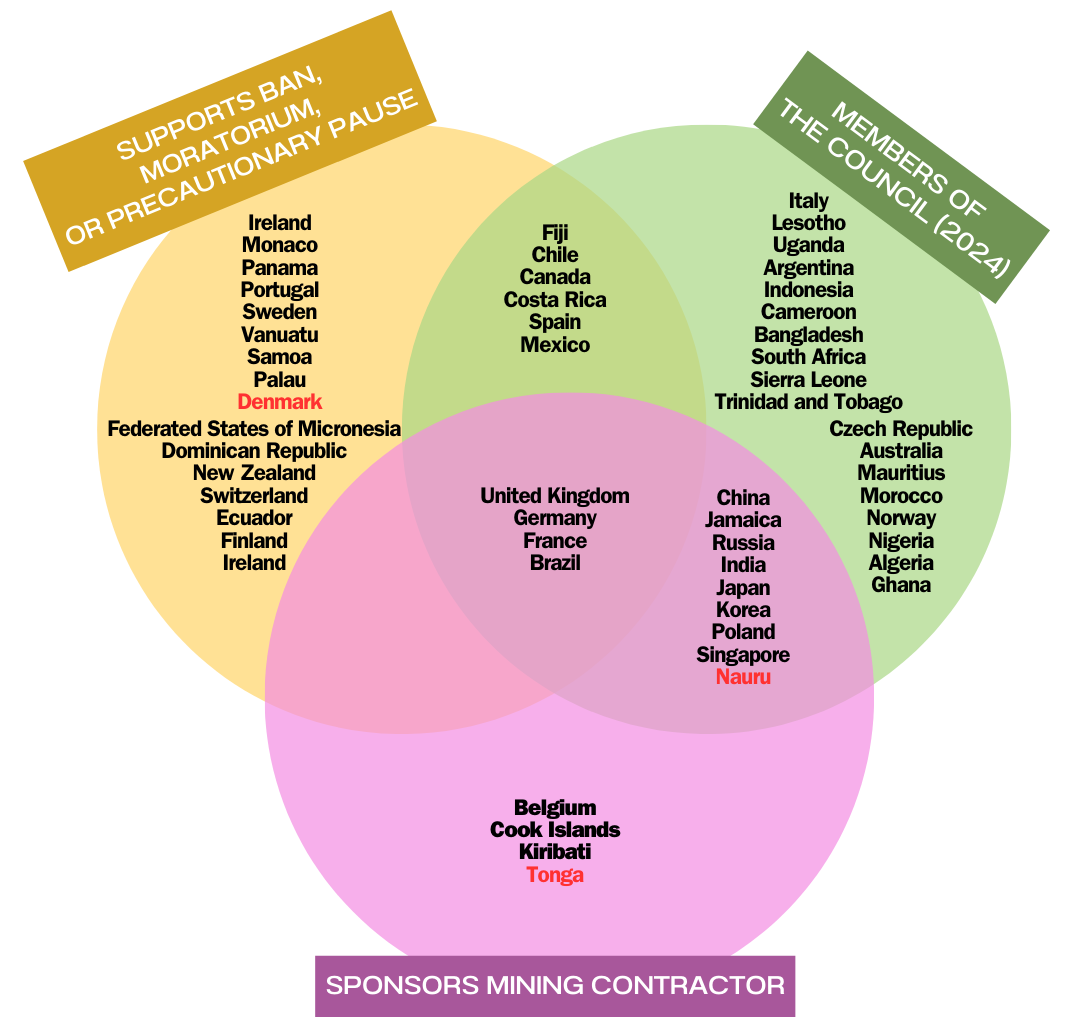
One curious contradiction in the International Seabed Authority is that some of the member states that are currently most vocal about enforcing a strong moratorium (if not outright ban) on deep-sea mining also currently hold ISA exploration leases. The UK and France, as well as Germany and Brazil, have all made statements in support of … Read More “What I’m watching for at this month’s ISA meeting: How are pro-moratorium member states dealing with their own mining leases?” »